NEWS
- The Application of Barium Sulfate in the Rubber Industry
- Effects of BaSO4 on the performance of valve-regulated lead-acid battery
- The salt lake is famous for its pure scenery, but it lasts for its precious deposits
- Why is nano-sized modified barium sulfate suitable for transparent filling masterbatch?
- Lack of Solution on Sampling?Professional Team Is Waiting for You to Pick!
- XRF Testing-A High Efficiency and Low Cost Prospecting Method
- Is petroleum actually extracted from rock?
- Exporting sluggish? 9X Minerals delivered Spain and Japan order at same time
- Breif Introduction of Barite Fluorite Dressing
- What will be the substitute of titanium dioxide, as it's classified as a carcinogen by EU
- Japanese Initial Order Successfully Delivered
- Comparison of Precipitated Barium Sulfate and Titanium Dioxide
- Brief Introduction of Fluorite Flotation Process
- Orders keep coming,9X Minerals exported barite to Europe again
- Do You Know How to Process Barite?
- Popular Science|Ten Applications of Barium Sulfate
- Popular Science | Barium Sulfate in Cosmetics
- 9X Minerals has established cooperation with domestic railway transport department
- Community Receives Donations from Enterprises for Poverty Alleviation
- More Than 6 Industries Relying On Barite
- Quick Domain Knowledge: 8 sheets to learn about barite
- Job Opening for Sales Engineer in Zambia
- European Client Visits Lijiashan Barite Mine
- 9X Minerals Finished Another Barite Export Achievement
- 800,000-ton Port landed in Dejiang County, Guizhou Province
- Environment and economy, we choose the balance
- Initial shipment of Chemical Grade Natural Barite has been delivered
- Impact of environmental protection policy on barite enterprises in 2018
- What is barium sulfate powder coating?
- Experts predict that the global barite consumption will reach 6.338 million tons in 2023, and prices will also keep increasing.
- The latest world oil reserves of the TOP12 countries came out, and where will the China's barite go in the future?
- Barite Mineral Processing
- Why does the superfine barium sulfate be the "Wing" of the coating industry?
Effects of BaSO4 on the performance of valve-regulated lead-acid battery

For valve regulated lead-acid batteries, several additives are often added to the negative plate in the production process to improve the charge discharge performance and battery life characteristics, such as barium sulfate, sodium lignosulfonate, acetylene black and so on. The selection of the above additives plays an important role in improving the performance of the battery. This paper will focus on one of them BaSO4.
Barium sulfate exists in the form of insoluble compound in the negative plate. Because the lattice structure of PbSO4 is very similar to that of BaSO4, the PbSO4 produced in the discharge process of negative plate will aggregate with BaSO4 as the crystal core, and the shape and distribution of PbSO4 are directly related to the characteristics of BaSO4. This paper will start from the characteristics of barium sulfate, and discuss its comprehensive influence on battery performance in detail.
1. Experiment
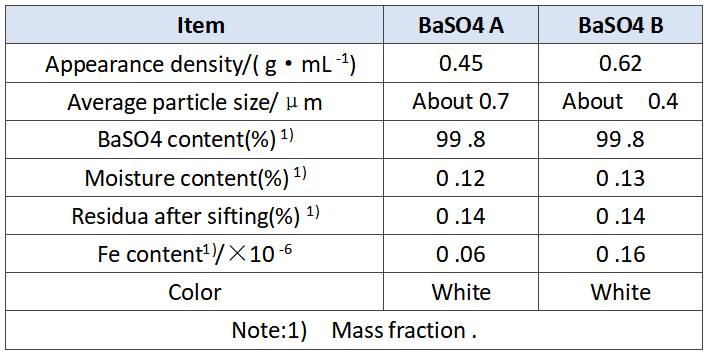
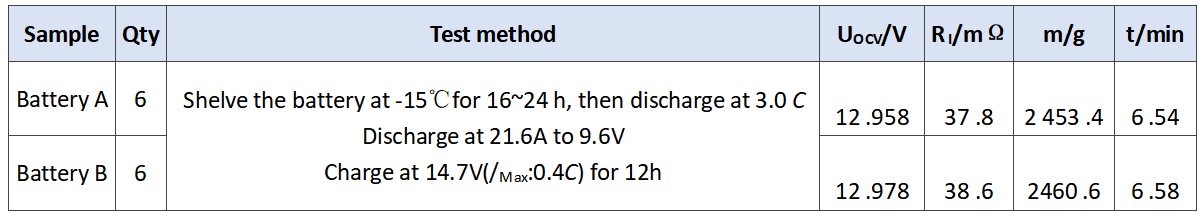
In order to verify the effect of barium sulfate on the performance of the battery, we selected A and B BaSO4 with different apparent densities for comparative test, as shown in Table 1. 12 V 7.2 ah battery was used in this test, and the difference is shown in Table 2. In addition to the different barium sulfate, the other raw materials, production conditions and parameters used in the experiment are completely the same, and the plate quality has been strictly screened, which effectively avoids the interference and influence of other factors, and ensures the uniformity and credibility of the experiment.
Tab .1 Characteristics of BaSO4 A and B
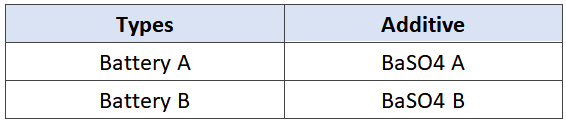
Tab.2 Types of experimental batteries
2. Results
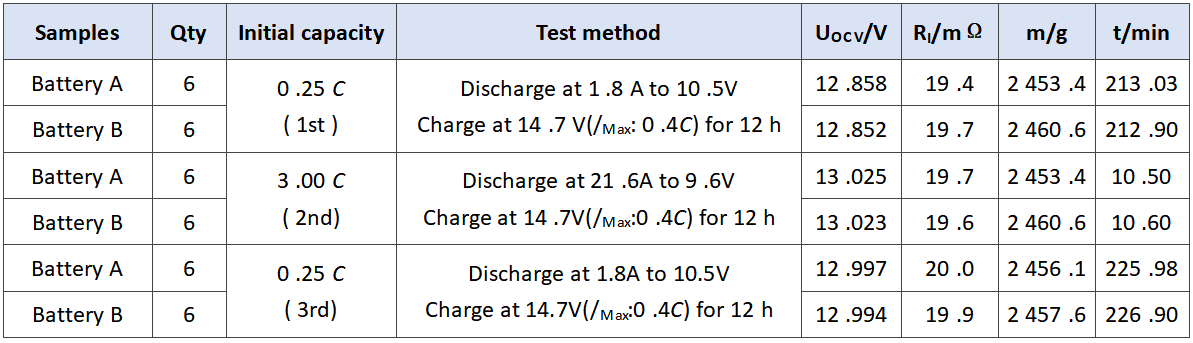
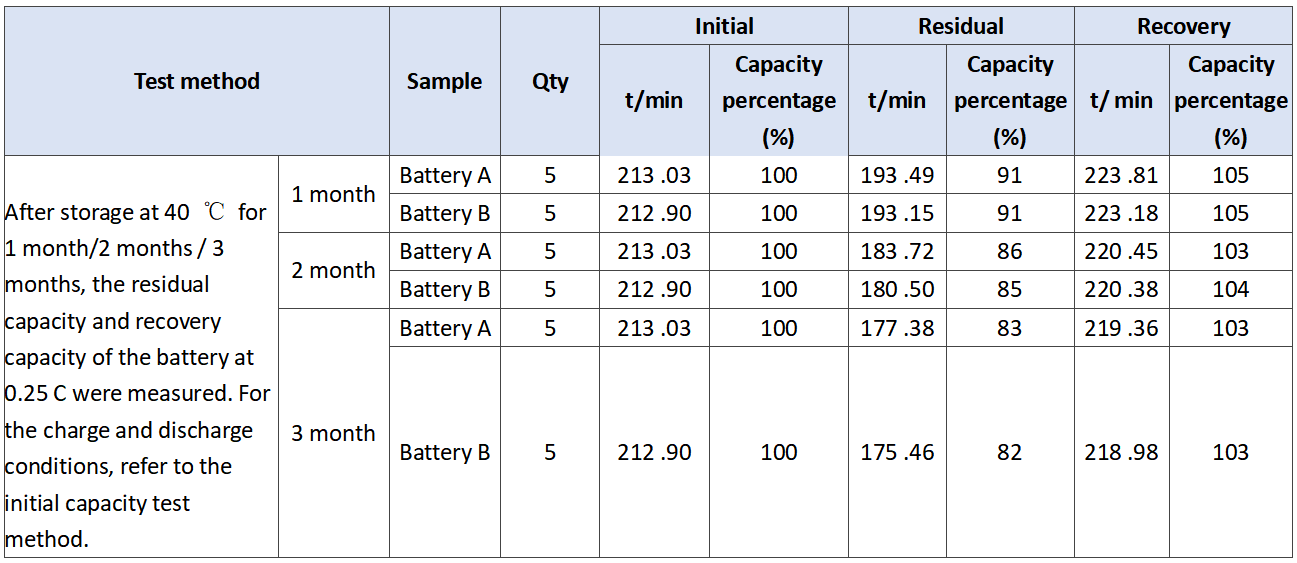
In order to verify the comprehensive effect of barium sulfate on the performance of battery, we evaluated the performance of battery a and battery B. the experimental results are as follows. The initial capacity is shown in Table 3. The discharge results at low temperature of 3.0 C are shown in Table 4. The storage experiment at 40℃ is shown in Table 5.
Tab.3 Initial capacity of experimental batteries
Tab .4 Discharge results of the experimental batteries at 3.0 C
Tab.5 Results of storage of the experimental batteries at 40 ℃
3. Analysis
The floating charge life test is mainly carried out according to the following methods: charging at constant voltage (13.7V) at 60℃, discharging at 3.0c and 0.25 C after 3 weeks, and charging at 13.7V (maximum current 0.2 C)×16 h. The above process is a cycle, such cycle, until the end of battery life. The experimental results are shown in Figure 1. The cycle life is shown in Figure 2.
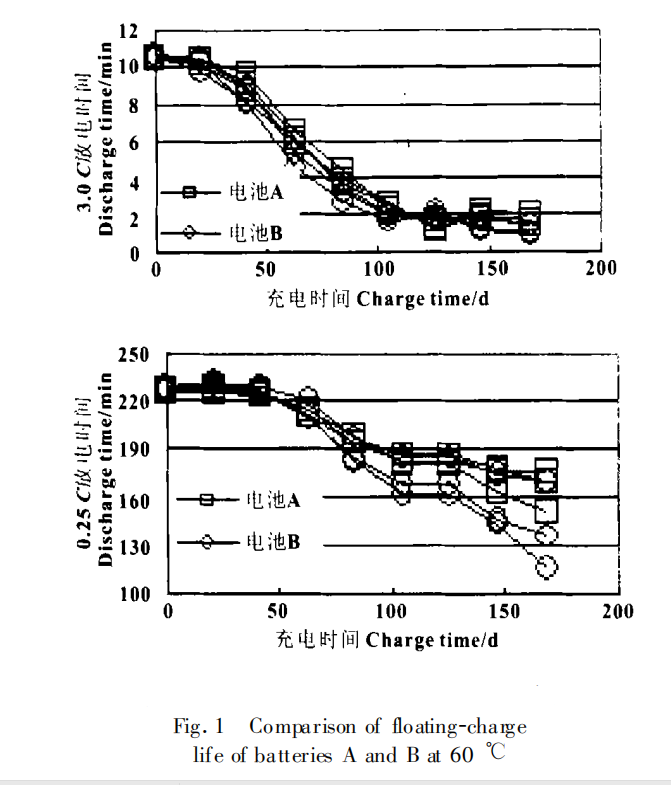
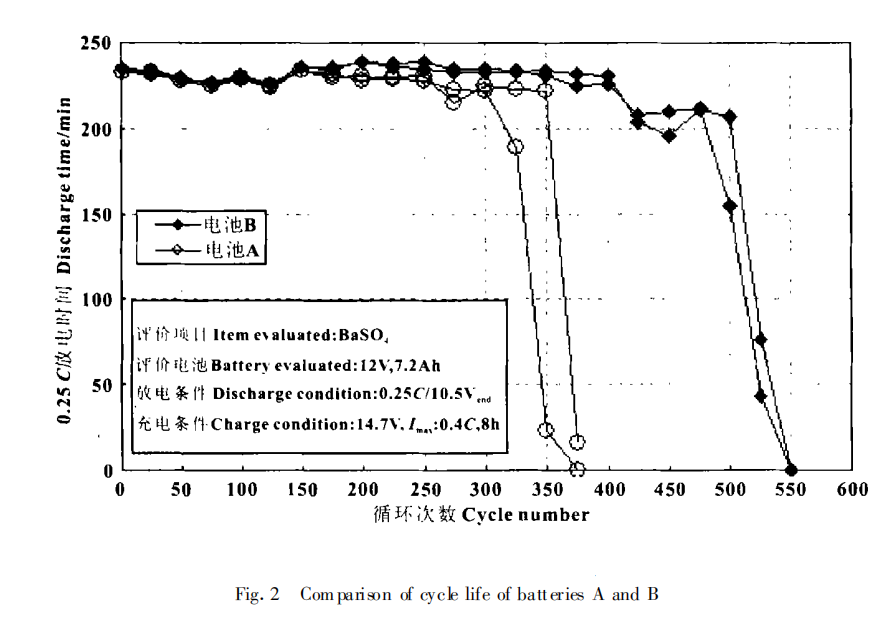
Let's start with sponge lead, the active material of negative plate. The lead sponge without recycling has a highly developed specific surface area of about 0.5-0.8 m2 / g and a porosity of about 50%. The active material in this state has a high surface energy, which is equal to the product of the real area of the negative plate and the surface tension (energy per unit surface). In thermodynamics, this high energy system is unstable and has a tendency of spontaneous change in the direction of energy decrease. When the battery is charged, the dissolution of PbSO4 is a process of Metal Electrodeposition by Pb2+ reduction, which provides the condition for the system to change to the direction of energy reduction. When the metal and solution system remains unchanged, the surface tension is basically constant, which can only reduce the surface area, even if the surface area shrinks, so that the energy of the system is reduced, which is manifested as tight junction of the negative plate, hardening and less pores. With the increase of battery cycle times, the active material of negative plate is constantly expanding and shrinking. Over time, the acceptance capacity of the negative plate will gradually decrease when charging at constant voltage, and a passivation layer will be formed, resulting in the deterioration of the contact surface between the grid and the active material, and eventually leading to the end of the battery life.
The addition of barium sulfate can effectively solve this problem. Barium sulfate has a lattice parameter similar to that of lead sulfate. The highly dispersed barium sulfate in the negative active material can be used as the crystallization center of lead sulfate during discharge. Since lead sulfate can crystallize and precipitate on isomorphic barium sulfate, there is no need to form lead sulfate crystal nucleus, so the supersaturation necessary for forming crystal nucleus will not occur. Under the condition of low supersaturation, PbSO4 is loose and porous, which is conducive to the diffusion of H2SO4 and reduces the concentration polarization. In addition, the existence of barium sulfate makes the product PbSO4 precipitate not on lead, but on barium sulfate, so that the active material lead is not covered by PbSO4 passivation layer, so barium sulfate plays a role in delaying passivation.

Moreover, barium sulfate can prevent the specific surface area of lead from shrinking during battery charging. Because barium sulfate is inert and does not take part in the redox process of the electrode, it is highly dispersed in the active material and mechanically separates lead from lead or lead sulfate from lead, so that it is not easy to merge between particles, so as to maintain the developed specific surface area of the active material of the negative plate. This is the reason why barium sulfate has a direct effect on the cycle life of the battery, but not on the initial capacity, low temperature high rate discharge, storage characteristics and floating charge life.
The apparent density of barium sulfate is different, and the average particle size is also different. Generally speaking, the higher the apparent density is, the smaller the average particle size is. When the input mass ratio is the same, the distribution of barium sulfate in the active material of the negative plate will be more uniform, and its effect on delaying the passivation of the active material of the negative plate and maintaining the specific surface area of the active material of the negative plate will be more obvious.
4. Conclusion
The above experiments show that barium sulfate has the following two functions:
(1) Barium sulfate can effectively prevent the surface area of the active material of the negative plate from shrinking during the cycle. It can be evenly distributed in the active material of the negative plate. By reducing the surface tension, the energy of the system is reduced, while the real surface area of the active material does not shrink;
(2) Barium sulfate can effectively prevent the passivation of the active material of the negative plate, which affects the PbSO4 structure formed in the discharge process of the active material of the negative plate. Barium sulfate has a significant effect on improving the cycle life of VRLA batteries, and the higher the apparent density is, the more obvious the effect is.
ABOUT US

9X Minerals LLC is an international mining company based in Southwestern China. To provide best and most suitable barite product to meet various clients’requirement is always our mission and responsibility.